In this example we can draw two Lewis structures that are energetically equivalent to each other that is they have the same types of bonds and the same types of formal charges on all of the structuresBoth structures 2 and 3 must be used to represent the molecules structureThe actual molecule is an average of structures 2 and 3 which are called resonance structures. The following is a general procedure for drawing Lewis structures.

How To Draw Lewis Structures For A Molecule With One Central Atom No Octet Rule Exceptions Chemistry Study Com
The molecular formula and the connectivity are determined by experiment.
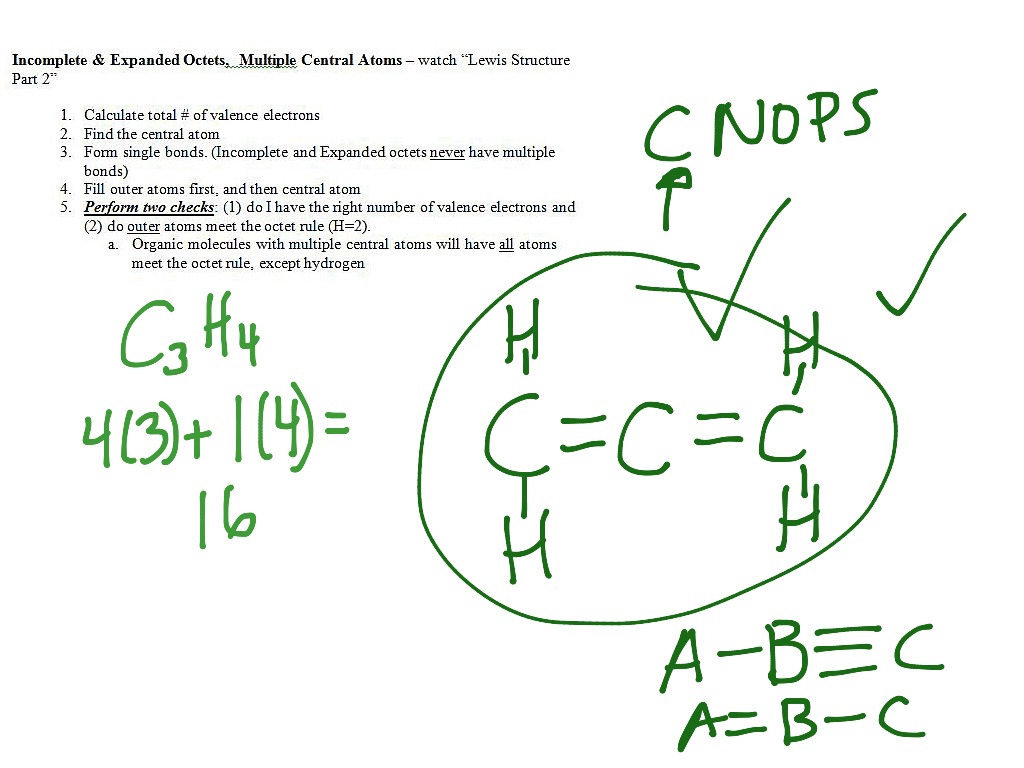
. Choose a central atom well start with small molecule examples for which there is only one central atom and the other atoms - the peripheral atoms - are all bonded to the central atom. Write the Lewis structure for CH2O where carbon is the central atom. CH 2 Br 2 d.
Electronegativity typically increases as you. Explore molecule shapes by building molecules in 3D. Find out by adding single double or triple bonds and lone pairs to the central atom.
If it has only one Lewis structure it doesnt have a resonance hybrid. Drawing Isomers of Organic Molecules. Central atom of SO 3 2-ion is sulfur.
Draw the Lewis dot structure for PF5 and provide the following information. PO 4 3 b. First determine the central atom.
Before we begin to learn the steps for drawing Lewis structure lets see the Lewis structure of IF3. This will be the atom with the lowest electronegativitySometimes its difficult to know which atom is the least electronegative but you can use the periodic table trends to help you out. As you already know valence electrons are used to draw the Lewis structures of any molecule.
The central atom bonds with each of the surrounding atoms which form bond angles of 1095. It will also work with more complex molecules and ions if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. H 2 S c.
Draw the Electron dot structure. The general rules for drawing Lewis structures are given below. The PH3 Lewis structure has 8.
Then compare the model to real molecules. CO 3 2 4. For the NO2 Lewis structure calculate the total number of valence electrons for the NO2 molecule.
In formic acid the carbon atom is a central atom. The Lewis structure of IF3 shows that I is surrounded by 2 lone pairs of electrons and forms 3 single bonds with each of the F atoms. Simple steps for drawing the Lewis dot structure for PF3.
Steps to Drawing a Lewis Structure. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO 3 2-ion.
Hydrogen H and fluorine F each have valence of 1 and generally these will. Sometimes there are several correct Lewis structures for a given molecule. How to Draw Lewis Structures.
Combine each atom with a single bond to the central atom by contributing one electron from each atom for the bond. Number of atoms bonded to the central atom b. For the following molecules or ions where the central atom is underlined.
How many electron groups are around the central atom. A Lewis structure also helps to make a prediction about the geometry of a molecule. Determine the total number of electrons available for bonding.
We still have 12 pairplace 3 pair on each terminal Cl atom. Hydrogen is the only exception to this since it forms only one bond. Count total valence electron in PF3.
416 The geometry of the central atoms and their non-bonding electron pairs in turn determine the geometry of the larger whole molecule. CCl4 Tally the valence electrons C 1 4 4 Cl 4 7 28 Total. Lewis Dot Structures Practice Sheet page 3 Steps for Drawing Lewis Dot Structures for Larger Molecules 1.
In the first step we have to find how many valence electrons are there in PF3 so that we can distribute them around central and terminal atoms with the goal of completing their octet shell. 32 valence electrons or 16 pair C is in center with 4 Cls aroundthis uses 8 electrons or 4 pair. Draw Lewis structures AND predict the molecular geometry of the following compounds or polyatomic ions.
Drawing the Lewis Structure for PH 3. Constitution The order in which the atoms of a. The compound is a chain of three oxygen atoms and minimizing the charges while giving each atom an octet of electrons requires that the central oxygen atom form a single bond with one terminal oxygen and a double bond with the other terminal oxygen.
Look up the electronegativity values for each element in your structure. For the PH3 Lewis structure we first count the valence electrons for the PH3 molecule using the periodic table. Table 14 How to Write Lewis Structures Step 1.
Pick a central atom. A Hydrogens H and halogens FClBrI are almost always outer atoms. Start your structure by picking a central atom and writing its element symbol.
In the Lewis structure for NO2 the Nitrogen atom is the least electronegative atom and goes at the center of the structure. After determining how many valence electrons there are in NO2 place them around the central atom to complete the octets. Number of lone electron pairs on.
NO 3 d. Draw the Lewis dot structure for each of the following polyatomic ions. The molecular geometry at the central carbon atom and hybridization of the central atom is trigonal planar and sp2 hybridization respectively.
They only want to form one bond to get to a noble gas configuration. Once we know how many valence electrons there are in PH3 we can distribute them around the central atom and attempt to fill the outer shells of each atom. Attached to a central atom is the one that has the maximum.
The least electronegative atom represents the central atom. A Lewis structure is a graphic representation of the electron distribution around atoms. All atoms except hydrogen follow the octet rule in the Lewis structure of formic acid.
Therefore five electron groups are around the central atom of. Methyl nitrite has the molecular formula CH3NO2. By In both Lewis structures there are eight lone pairs on all oxygen atoms.
How does molecule shape change with different numbers of bonds and electron pairs. The first step of drawing resonance structures starts with drawing all the possible Lewis structures. Ozone O 3 O_3 O 3 is one example.
NH 4 c. Draw the Lewis dot structures for each of the following molecules. In drawing Lewis structures for relatively small molecules and polyatomic ions the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms.
The number of electron pairs in the valence shell of a central atom is determined after drawing the Lewis structure of the molecule and expanding it to show all bonding groups and lone pairs of electrons.
Chem 101 Octet Rule Violations

Lewis Structures Multiple Central Atoms Science Chemistry Chemical Bonds Showme

Writing Lewis Structures Ppt Video Online Download
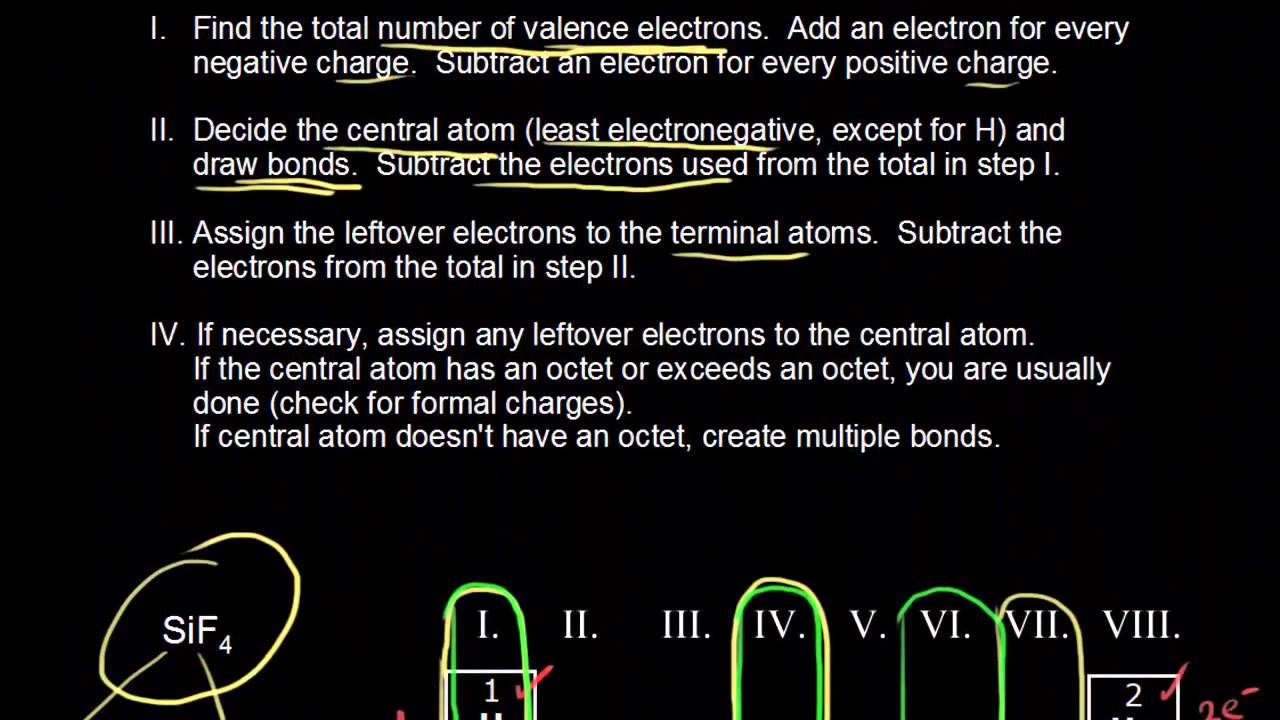
Drawing Dot Structures Video Khan Academy

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421
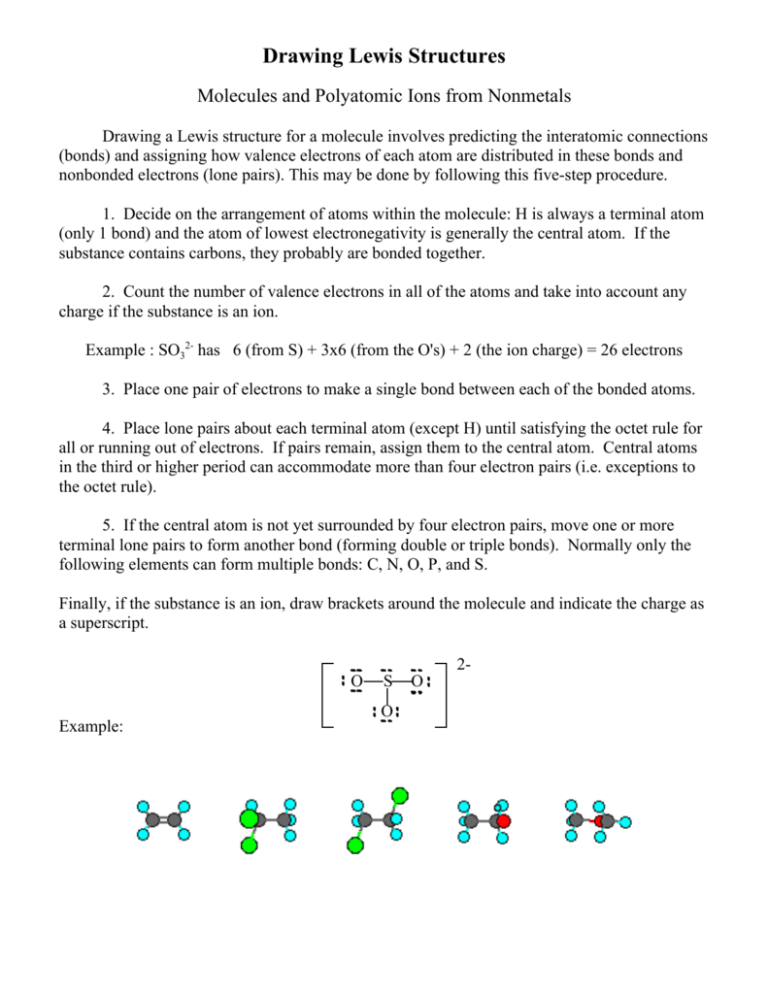
Rules For Drawing Lewis Structures

Step 1 Identify The Central Atom And Draw It S Lewis Structure This Is The Element With The Lowest Number Of Atoms In The Formula Draw A Lewis Dot Diagram Ppt Download

0 comments
Post a Comment